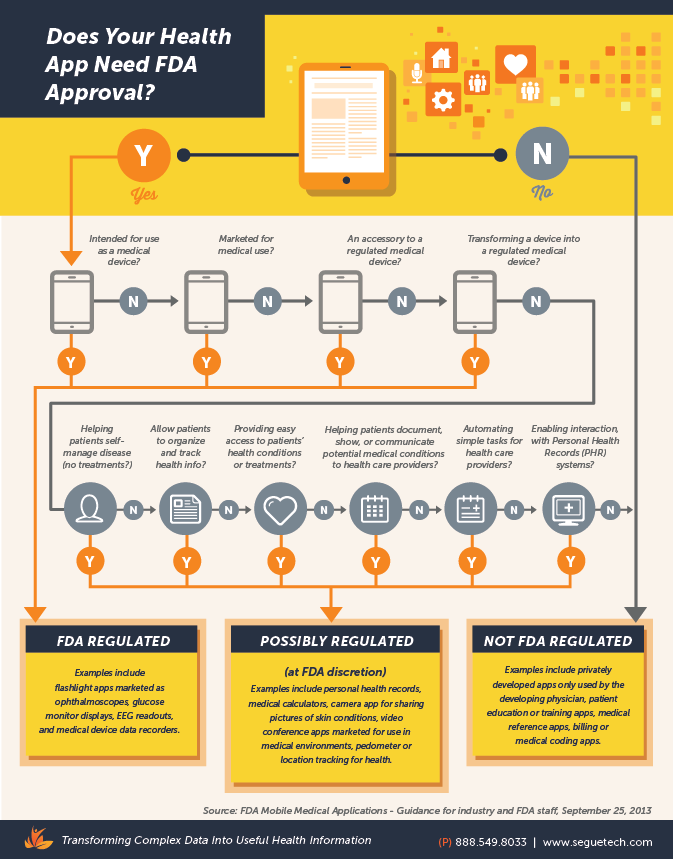
On September 21, the U.S. Food and Drug Administration (FDA) issued its long awaited final guidance for developers of mobile medical applications. The FDA stated that it intends to exercise enforcement discretion for the majority of mobile apps as they pose minimal risk to consumers. The FDA’s current guidance indicates that the majority of medical apps are either:
- Not medical devices as defined by the Federal Food, Drug and Cosmetic Act (FD&C) and therefore do not fall under FDA regulation, or
- If defined as medical devices, pose a risk level low enough to preclude enforcing FD&C requirements.
The Agency is attempting to focus regulatory oversight on a subset of mobile medical apps that present a greater risk to patients if they do not work as intended, while continuing to encourage innovation in the field of medical mobile app development. The primary areas of this oversight are for mobile apps that:
- Are intended to be used as an accessory to a regulated medical device (example: an app that allows health care professionals to make a specific diagnosis by viewing a medical images from a specific apps operating on a smartphone or a mobile tablet) and
- Transform a mobile platform into a regulated medical device (example: an application that turns a smartphone into an electrocardiography (ECG) machine to monitor heart rhythms)
The FDA is trying to take a careful approach to a technology that is growing exponentially and transforming constantly. It seems reasonable to regulate technology that might pose a risk to patient health if it malfunctions or is used improperly, yet no one wants to see the FDA begin “regulating” how smartphones and tablets are produced and sold. The current guidance is trying to find that balance. Need more clarity? Check out our infographic below.